Desensitization in gene therapy
Currently, it is estimated that antibodies prevent up to 1 in 3 people from benefiting from gene therapy treatments.1-4
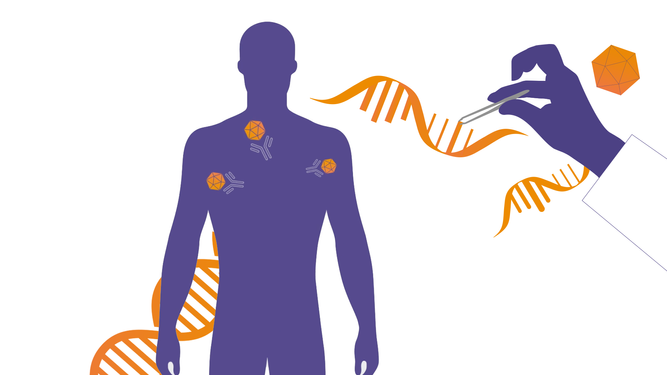
For people living with rare, monogenic diseases, gene therapies may offer a cure to a life-long condition by introducing genetic material that compensates for a defective gene. However, if the therapy is based on the use of Adeno Associated Viruses (AAV) vectors, the immune system may carry antibodies that counteract the gene therapy treatment preventing its success.2,5-6 Understanding how to overcome the action of anti-AAV antibodies is crucial to ensure all patients in need can receive gene therapy treatments.
Addressing barriers to gene therapy treatment
For people living with rare, monogenic diseases, gene therapies may offer a cure to a life-long condition by introducing genetic material that compensates for a defective gene.
Our commitment
Bridging the access to gene therapies for more patients
We are collaborating with gene therapy companies at the forefront of innovation to evaluate the potential of our technology as a pre-treatment to gene therapy. The hope is that by creating an IgG-free window in those patients with anti-AAV antibodies, it would allow for the successful administration of a gene therapy treatment.
Sarepta Therapeutics

We work with Sarepta Therapeutics to develop imlifidase as a pre-treatment prior to the administration of gene therapy for Duchenne muscular dystrophy and Limb-girdle muscular dystrophy in patients with antibodies targeting adeno-associated virus (AAV).
AskBio
With AskBio we are evaluating the use of imlifidase as a pre-treatment prior to the administration of gene therapy in Pompe disease for patients with anti-adeno-associated virus (AAV) antibodies.
Généthon

We work with Généthon to assess the use of imlifidase as a pre-treatment preceding the administration of Généthon’s gene therapy in Crigler-Najjar syndrome in patients with antibodies targeting adeno-associated virus (AAV). The collaboration, set as a preclinical and clinical feasibility program, evaluates the safety and efficacy of imlifidase in this setting.
References
1. Boutin S, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010 Jun;21(6):704-12. doi: 10.1089/hum.2009.182. PMID: 20095819.
2. Calcedo R, Wilson JM. Humoral Immune Response to AAV. Front Immunol. 2013 Oct 18;4:341. doi: 10.3389/fimmu.2013.00341. PMID: 24151496; PMCID: PMC3799231.
3. Veron P, Leborgne C, Monteilhet V, Boutin S, Martin S, Moullier P, Masurier C. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol. 2012 Jun 15;188(12):6418-24. doi: 10.4049/jimmunol.1200620. Epub 2012 May 16. PMID: 22593612.
4. Kruzik A, et al. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol Ther Methods Clin Dev. 2019 Jun 7;14:126-133. doi: 10.1016/j.omtm.2019.05.014. PMID: 31338384; PMCID: PMC6629972.
5. Falese L, et al. Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017 Dec;24(12):768-778. doi: 10.1038/gt.2017.95. Epub 2017 Nov 6. PMID: 29106404; PMCID: PMC5746592.
6. Leborgne C, et al. IgG-cleaving endopeptidase enables in vivo gene therapy in the presence of anti-AAV neutralizing antibodies. Nat Med. 2020 Jul;26(7):1096-1101. doi: 10.1038/s41591-020-0911-7. Epub 2020 Jun 1. PMID: 32483358.